Why is Roman concrete more durable than modern concrete?
Modern concrete is porous and degrades in contact with seawater. Seawater can seep into its pores, and when dried out the salts crystalize. The crystallization pressure of the salts produces stresses that can result in cracks and spalls. There are also other chemical processes such as sulphate attack, lime leaching and alkali-aggregate expansion all of which degrade modern concrete. Some submerged concrete objects may last only 10 years; meanwhile, 2000-year old concrete constructed during the Roman Empire is still going strong (Figure 1). Why this is so is a question an international research team led by geologist Marie Jackson of Utah University sought to reveal.

The composition of Roman concrete has been long known, being a mixture of volcanic ash, quicklime (calcium oxide) and volcanic rock, but the science behind its resilience to seawater remained unknown until recently. It is thought volcanic material was used after the Romans observed ash from volcanic eruptions crystallize to form durable rock.
The research team discovered that while modern concrete is made to be inert, the Roman version interacts with the environment. When seawater interacts with the mixture, it forms rare minerals aluminous tobermorite and phillipsite which are believed to strengthen the material. This discovery could lead to the development of more resilient concrete to be used in coastal environments.
Modern concrete is generally limestone mixed with other ingredients such as sandstone, ash, chalk, iron and clay. The mixture is designed to be inert and not interact with the environment. In coastal environments building regulations govern the type of concrete used and water-cement ratio, but the concrete is still porous: seawater can pass through the material, leading to corrosion and destructuralisation.
As well as salt crystallization, the process whereby dried out salts within the concrete lead to a buildup of pressure, other chemical reactions can affect the integrity of concrete. These include sulphate attack, lime leaching and alkali-aggregate expansion (Figure 2). Sulphate attack occurs when sulphates in the water react with the hydrated calcium aluminate within the concrete. This changes the microstructure and leads to an increase in volume within the concrete, resulting in physical stress and potential cracking. Lime leaching is the simple process of water passing through the concrete and dissolving calcium hydroxide from the concrete. (Calcium hydroxide is formed from the action of calcium oxide and water.) This is often seen as white patches or stalactites on the exterior of the concrete and reduces its strength. Alkali-aggregate expansion is when aggregates, such as silica, decrease the alkalinity of the cement paste, resulting in the expansion of minerals and cracking of the cement.

Roman concrete however does not appear susceptible to any of these processes. The research team found that seawater, the kryptonite to modern concrete, was the magic ingredient responsible for the structural stability of the Roman mixture. The Roman concrete samples were found to contain rare aluminous tobermorite and phillipsite crystals. It is believed that with long-term exposure to seawater, tobermorite crystalizes from the phillipsite as it becomes more alkaline. This crystallization is thought to strengthen the compound, as tobermorite has long plate-like crystals that allow the material to bend rather than crack under stress. Pliny the Elder in the first century CE exclaimed “that as soon as it [concrete] comes into contact with the waves of the sea and is submerged [it] becomes a single stone mass (fierem unum lapidem), impregnable to the waves and every day stronger.”

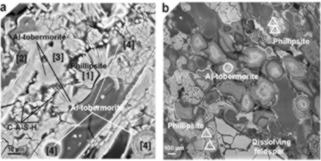
To arrive at these conclusions, Jackson et. al. (2017) performed scanning electron microscopy (SEM), micro x-ray diffraction (XRD), Raman spectroscopy and electron probe microanalysis at the Advanced Light Source at the Lawrence Berkeley National Laboratory. Samples were obtained by drilling Roman harbour structures, and were compared with volcanic rock (Figure 3). The combination of these techniques in conjunction with in situ analysis provided evidence of crystallized aluminous tobermorite and phillipsite within Roman marine concrete (Figure 4). These crystals formed long after the original setting of the concrete. This finding was surprising, as tobermorite typically forms only at temperatures above 80 °C, though there is one occurrence of it forming at ambient temperature in the Surtsey volcano.
After this discovery, there is now a desire to develop a concrete mixture which replicates ancient Roman marine concrete. It could result in more environmentally friendly concrete construction, and would provide a mixture resilient to seawater and advantageous to coastal defence.
References
Jackson, M.D. et. al. (2017). Phillipsite and Al-tobermorite mineral cements produced through low-temperature water-rock reactions in Roman marine concrete. American Mineralogist: Journal of Earth and Planetary Materials, 102(7), pp.1435-1450.
Jackson, M.D. et. al. (2013). Unlocking the secrets of Al-tobermorite in Roman seawater concrete. American Mineralogist, 98(10), pp.1669-1687.
Suprenant, B.A. (1991). Designing concrete for exposure to seawater. Concrete Construction Magazine, pp.814-816.
About the author/s

Jacob Evans
Jacob has been involved in the development of our FloodAUS, CyclAUS and QuakeAUS Cat-Loss models. He specialises in data science and mathematics.
Jacob received his PhD in Condensed Matter Physics from Macquarie University. Jacob’s interests include data science, numerical modelling, physics and mathematics.
Joining Risk Frontiers in 2017, Jacob has worked across a range of projects from model development and climate risk management to resilience and portfolio modelling.
Notably, Jacob has employed techniques such as machine learning and statistical analysis to understand the vulnerability of risk across Risk Frontiers catastrophe suite, as well as applying machine learning algorithms for flood, cyclone and earthquake models. He also works on hazard and loss modelling.
